· Hydrogen peroxide is a chemical compound containing molecules of hydrogen and water Its · The products are Hydrogen peroxide, and water Peroxide salts do occur, the peroxide anion is ()OO() So Sodium peroxide has the formula Na2O2 Generally, peroxide salts are found by burning Group I and Group II metals in air Metal peroxides (well, group I and II) can oxidize organic molecules to carbonatesNot to be confused with Trioxane Trioxidane (also systematically named μtrioxidanediidodihydrogen), also called hydrogen trioxide or dihydrogen trioxide, is an inorganic compound with the chemical formula H O 3H (also written as H (μO 3)H or H

Lewis Structure Hydrogen Peroxide Molecule Structural Formula Laughing Gas Structure Angle Text Png Pngegg
How to write the formula for hydrogen peroxide
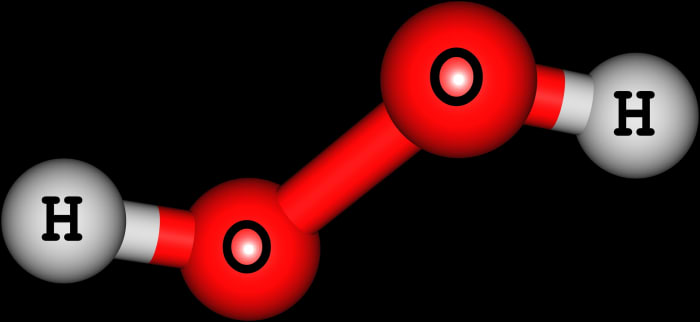
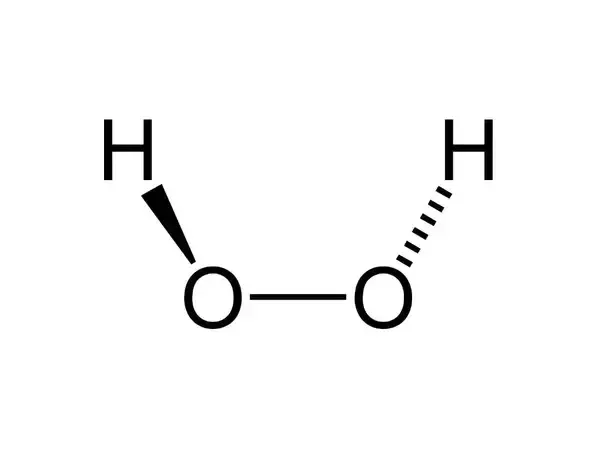
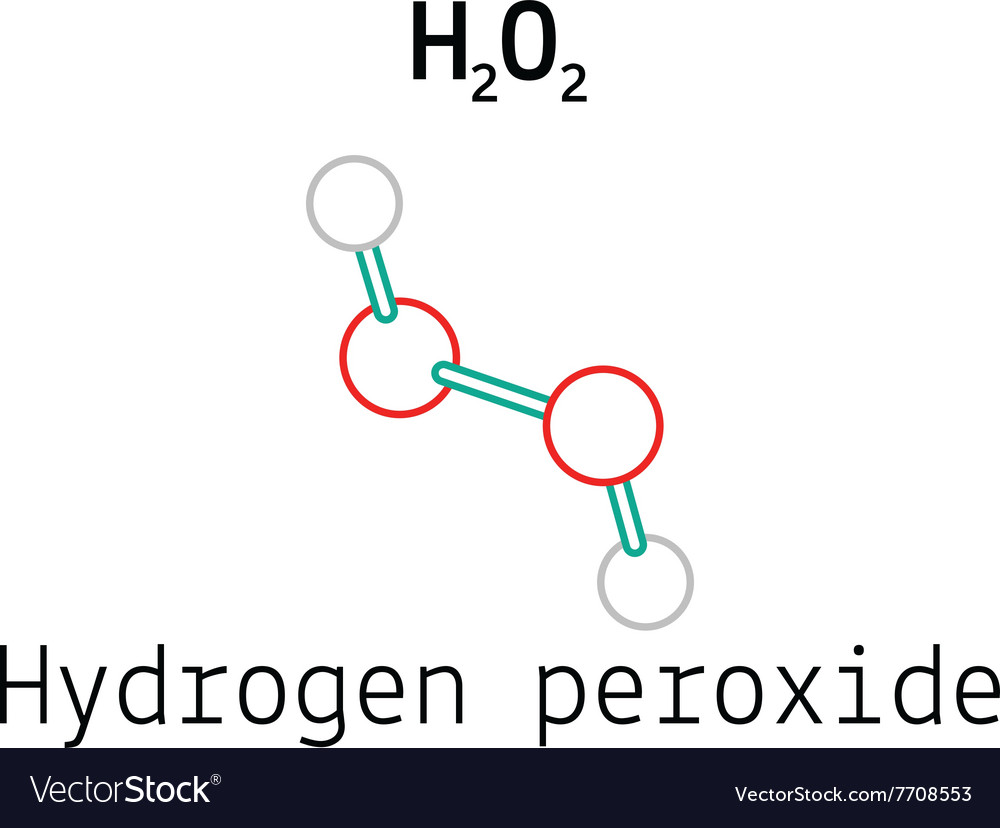
How to write the formula for hydrogen peroxide-Hydrogen peroxide has the chemical formula H 2 O 2 and the following structural formula HOOH The hydrogen peroxide molecule contains one extra oxygen atom, compared to the more stable water molecule The bond between the two oxygen atoms, the socalled peroxide bond, is broken while two HO radicals are formed · Benefits Of Hydrogen Peroxide – Remove Discolorations And Blemishes Using hydrogen peroxide is an excellent remedy for fading away acne scars, age spots, dark spots, and other discolorations 4 For this treatment, take a little hydrogen peroxide and use a Qtip to apply it to these areas Keep it on for ten minutes before rinsing it off



Why Hydrogen Peroxide Is A Polar Molecule Yvbi2pxx Chemistry Topperlearning Com
Solid state structure Geometry of hydrogen 1 coordinate terminus Prototypical structure Element analysis The table shows element percentages for H 2 O 2 (hydrogen peroxide)Heterolytic cleavage of bound hydrogen peroxide produces a water molecule and generates a compound I intermediate, which is two oxidation states above the ferric resting state Compound I is a highly oxidizing Fe IV O or ferryl species with either a porphyrin πcation radical or a cationic Trp radical Compound I undergoes a one electronHydrogen peroxide does not induce significant changes in tooth enamel organic and inorganic relative contents, and it whitens teeth just by oxidizing their organic matrix These findings are of great clinical significance since they explain the mechanism of tooth bleaching, and help understanding it
8 rows · Hydrogen peroxide H2O2 CID 784 structure, chemical names, physical and chemical · Hydrogen peroxide molecule has molecular formula H2O2 having an interesting skew structural unit It is used widely in industrial solutions and antiseptic or mouth cleaner in medicine Due to the high oxidizing properties, it is used as a bleaching agentHydrogen Peroxide (H 2 O 2) Lewis Structure Lewis structure of Hydrogen peroxide (H 2 O 2) contains two OH bonds and one OO bond Also, there are two lone pairs on each oxygen atom Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2
· Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant inHydrogen peroxide, (H 2 O 2 ), a colourless liquid usually produced as aqueous solutions of various strengths, used principally for bleaching cotton and other textiles and wood pulp, in the manufacture of other chemicals, as a rocket propellant, and for cosmetic and medicinal purposes Solutions containing more than about 8 percent hydrogen peroxide are corrosive to the skin · This activity will help assess your knowledge of the structure, uses, and formula of the bleaching agent called sodium hypochlorite Hydrogen Peroxide Preparation, Properties & Structure



Hydrogen Peroxide 30 32 W W 100 Volumes Certified Ar For Analysis Fisher Chemical Home Fisher Scientific


Molecules And Ions
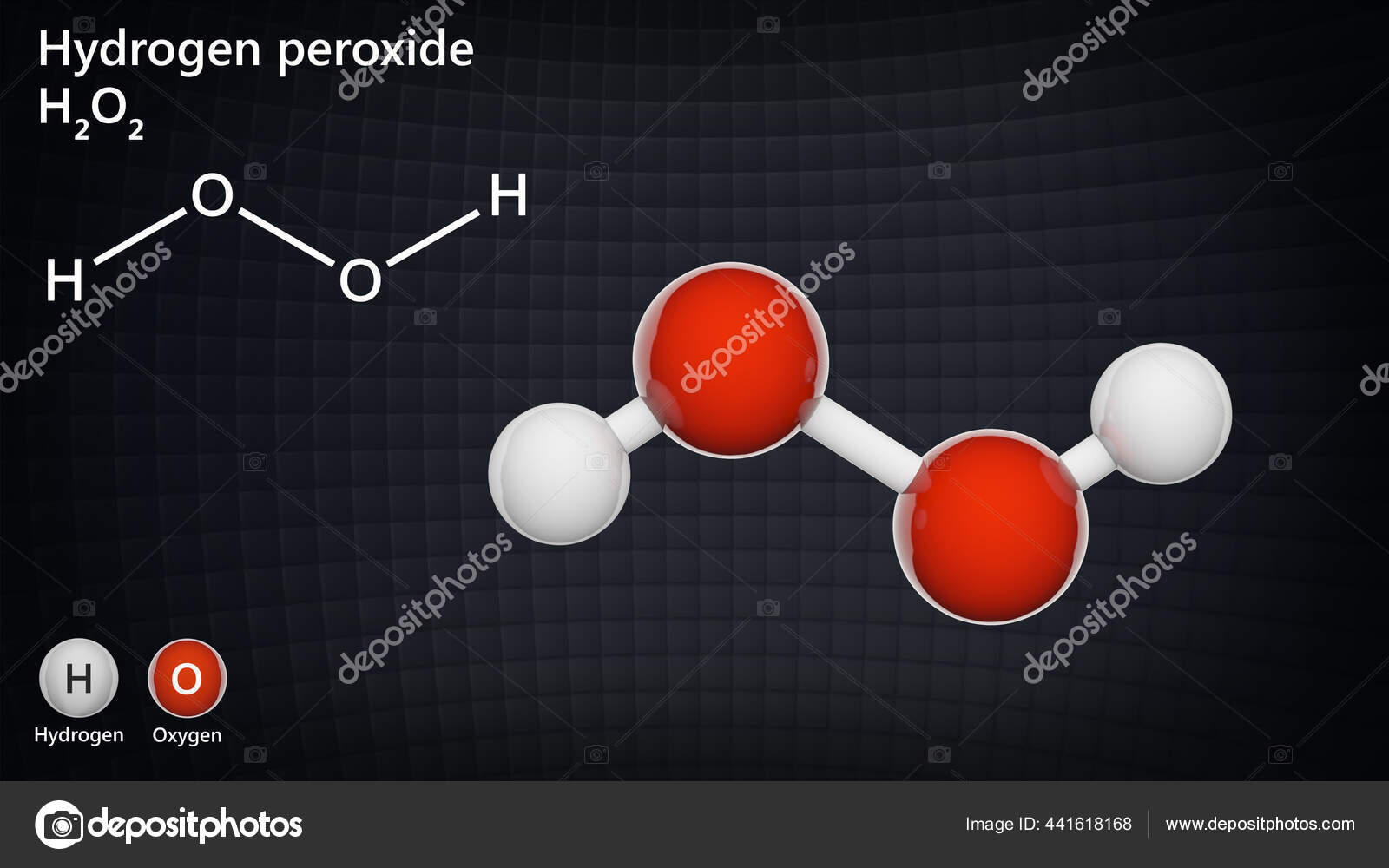
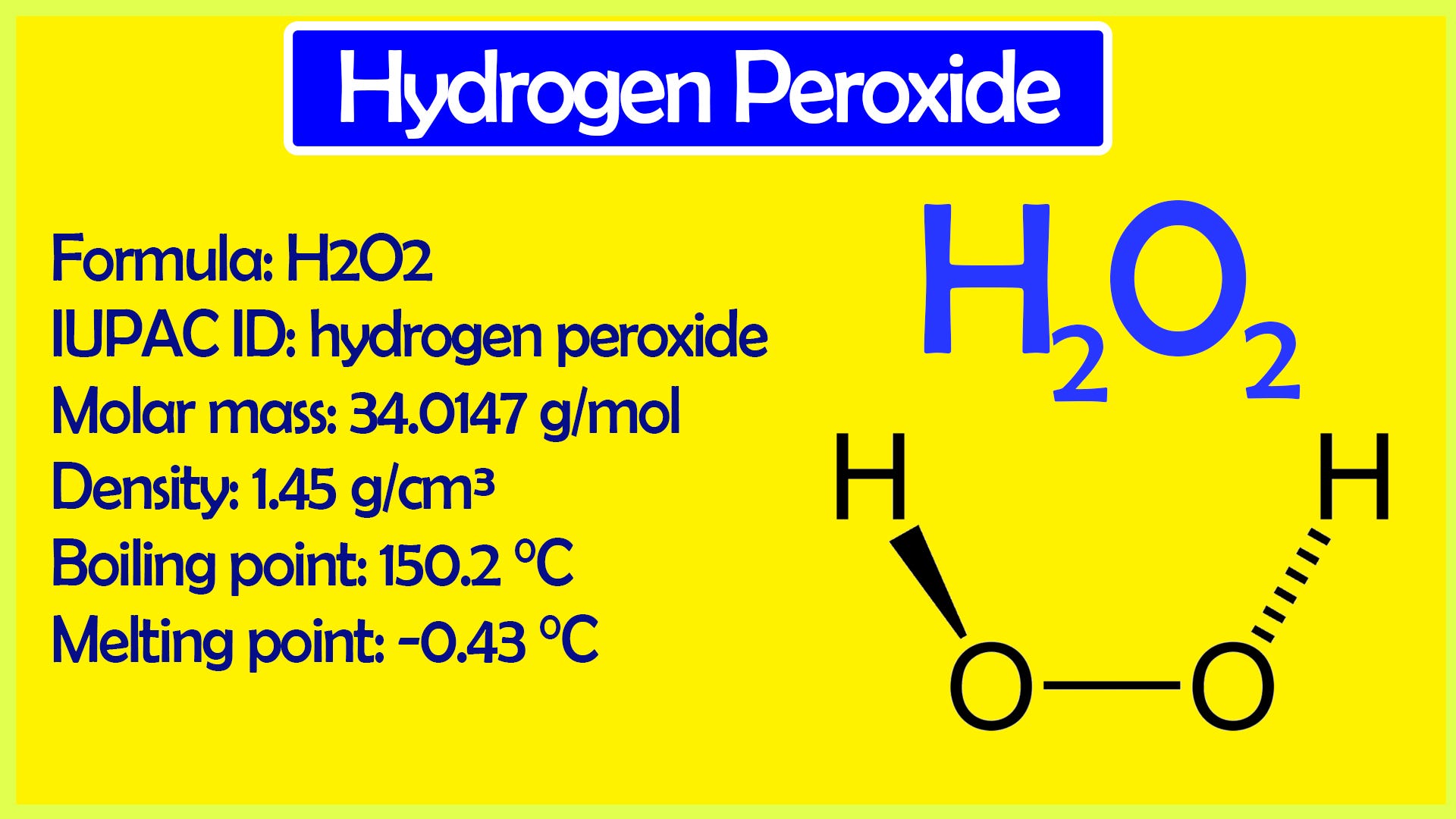
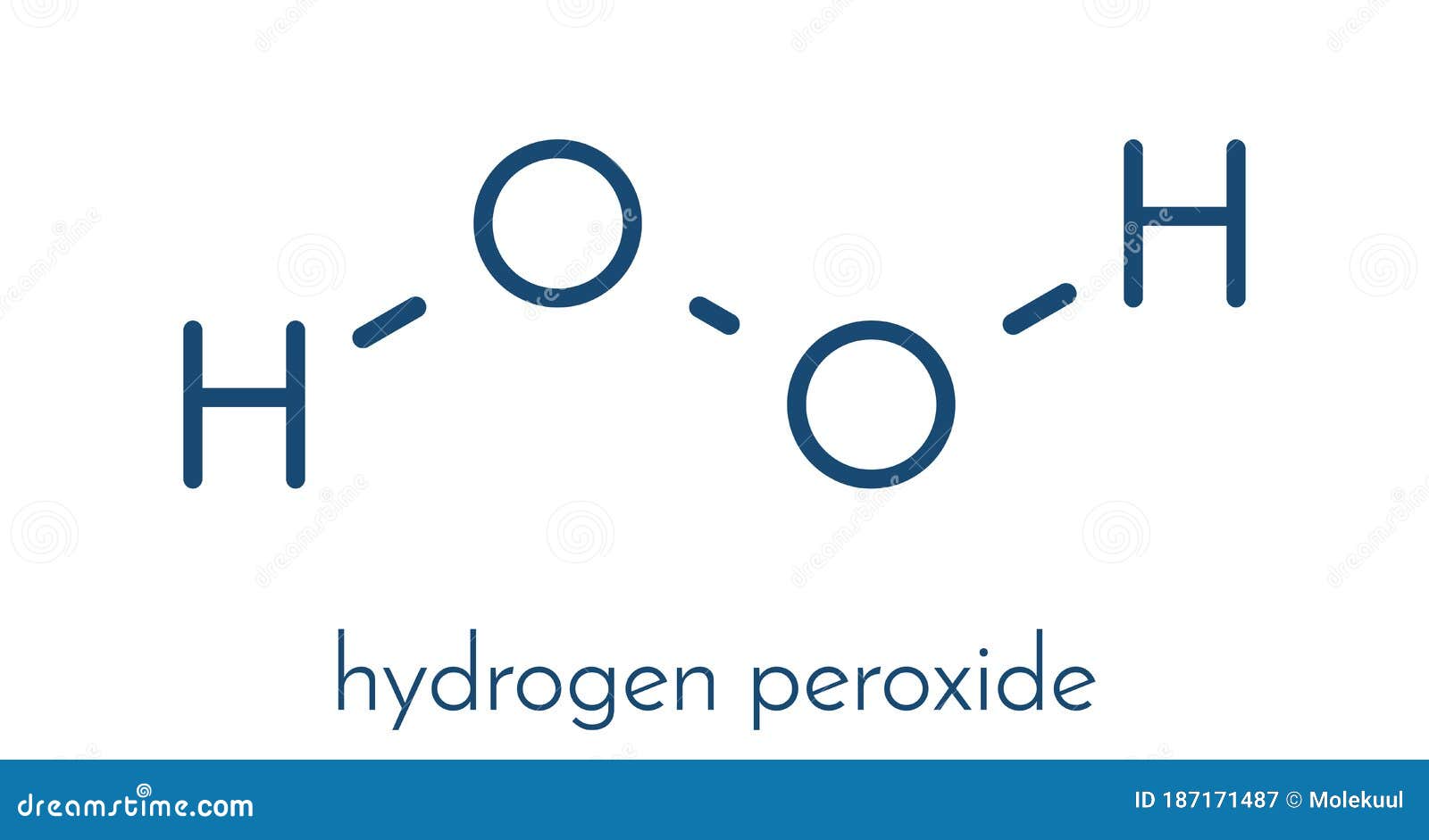
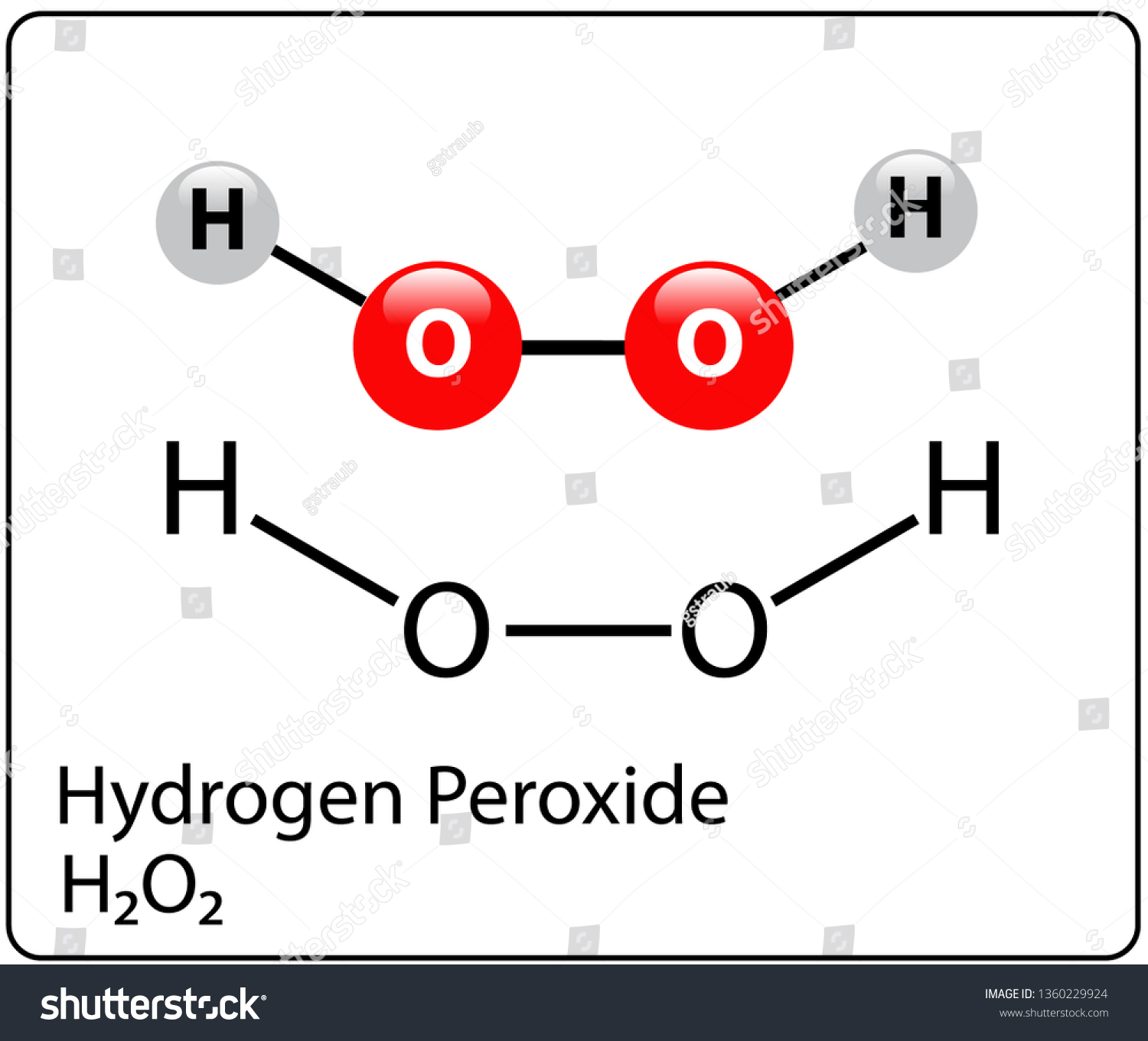
· Hydrogen Peroxide – also known by its chemical formula, H2O2 – is a powerful oxidising agent composed of water and oxygen When using a 35% foodgrade source with no additives or impurities, Hydrogen Peroxide can be miraculous in helping toThe molecular formula of hydrogen peroxide is H 2 O 2 (also written as HOOH) The 'H' stands for hydrogen, and the 'O' stands for oxygen This means that there are two hydrogen atoms and two oxygen atoms per molecule Boiling Point Of Hydrogen PeroxideThe OO group is the peroxide group of the compound And Hydrogen Peroxide is the simplest peroxide The chemical formula for hydrogen peroxide is H2O2 It is a water molecule with one extra atom of oxygen, It has various uses ranging from disinfectant to propellant for rockets



Urea Hydrogen Peroxide Sigma Aldrich


Why Is Hydrogen Peroxide Kept In Colored Bottles Class 11 Chemistry Cbse
· Hydrogen peroxide H2O2 CID structure, chemical names, physical and chemical Hydrogen peroxide 3D structure DOT Emergency GuidelinesIts structure is HOOH, with the peroxide OO group right in there between the hydrogens Hydrogen peroxide in the form of carbamide peroxide is widely used for tooth whitening (bleaching), both inComplete the electronic structure of sodium (2) Page 3 of 10 (b)€€€€ The chemical equation for a reaction of sodium is shown below Q4€€€€€€€€€ The formula for the compound hydrogen peroxide is H 2 O 2Urea hydrogen peroxide (CHEBI) has part hydrogen peroxide (CHEBI) hydrogenperoxide(1−) (CHEBI) is conjugate base of hydrogen peroxide (CHEBI)



Hydrogen Peroxide Nerdy Jokes



Hydrogen Peroxide Formula Uses Britannica
Correcting an error in the last video regarding hydrogen peroxideMolecular Formula H4O3 Synonyms hydrogen peroxide water H2O2 H2O Molecular Weight 53 g/mol Component Compounds CID 784 (Hydrogen peroxide)Structure, properties, spectra, suppliers and links for Hydrogen peroxide urea, , CARBAMIDE PEROXIDE



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Stock Photo Picture And Royalty Free Image Image



15 Hydrogen Peroxide Structural Formula Stock Photos Pictures Royalty Free Images
Boiling points of hydrogen peroxide and water are 150 0 C and 100 0 C respectively We know both hydrogen peroxide and water are able to make hydrogen bonds · Hydrogen peroxide is an approved EU basic/commodity susbstance It is highly soluble in water and in its pure state it is highly volatile but much less so diluted It is not environmentally persistent Hydrogen peroxide is not expected to have an immediate or delayed harmful effect on human or animal health(R)6hydroxynicotine oxygen H2O > 6hydroxypseudooxynicotine hydrogen peroxide PlantCyc HYDROGENPEROXIDE, HYDROGENPEROXIDE, HYDROGENPEROXIDE, HYDROGENPEROXIDE, HYDROGENPEROXIDE, HYDROGENPEROXIDE (S)6hydroxynicotine oxygen H2O > 6hydroxypseudooxynicotine hydrogen peroxide PlantCyc HYDROGENPEROXIDE, HYDROGENPEROXIDE


Hydrogen Peroxide Structure Line Icon Stock Vector Illustration Of Outline Bleach


Hydrogen Peroxide Molecule Of The Month September 06 Html Version
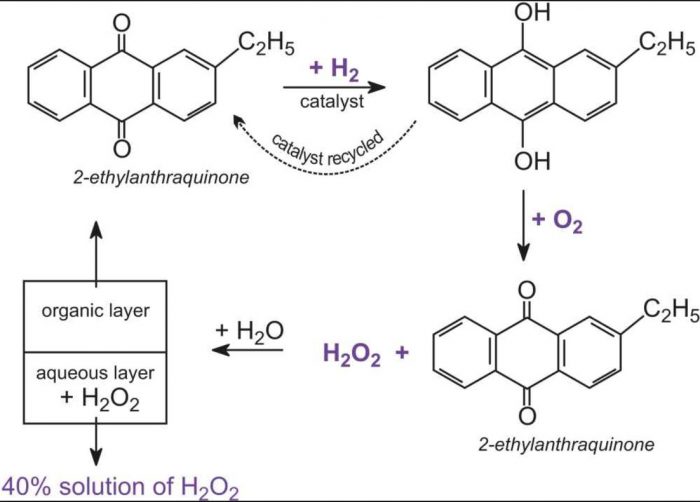
· Hydrogen Peroxide Structure, Uses and Properties Hydrogen peroxide is a highly unstable chemical compound Two molecules of hydrogen combine with two molecules of oxygen to form hydrogen peroxide Hence, its chemical formula is H 2 O 2Formula and structure Hydrogen peroxide has the chemical formula H 2 O 2 Its molecular formula is H 2 O 2 and its molar mass is g mol 1 · INTRODUCTION Hydrogen Peroxide was discovered by a French chemist JL Thenard in 1818 Its molecular formula is H2O2 3 PREPARATION OF H2O2 H2O2 can be prepared in laboratory by 1 The action of cold, dilute sulphuric acid on sodium 2 The action of cold, dilute sulphuric acid on barium peroxide 4 PREPARATION OF H2O2 1



Hydrogen Peroxide Urea Wikipedia


Structural Chemical Formula Molecular Structure Hydrogen Peroxide H2o2 Chemical Structure Stock Photo Image By C Orangedeerstudio
Hydrogen peroxide is a highly reactive chemical containing the elements hydrogen and oxygen (H2O2) Pure hydrogen peroxide is a colourless liquid, but it is sold on the market as solutions in water, containing up to 33 – 37% pure hydrogen peroxide and other additives to prevent product decomposition In industry, it is mainly used in the production of chemicals and in bleaching ofHydrogen peroxide is an oxidizing agent that can be used as a highlevel disinfectant 27 It produces destructive hydroxyl radicals that attack membrane lipids, DNA, and other cellular components when used at recommended concentrations 27 Its antimicrobial activity is very slow 27,28 It is marketed as Sporox as a premix that contains 75% hydrogen peroxide and 085%The Hydrogen Peroxide Breakdown 330 Laying the Foundation in Biology 7 Enzymes work by lowering the energy of activation For example, hydrogen peroxide decomposes to form water, H2O, and oxygen gas, O2 While this is a catabolic reaction, the rate at which it occurs is slow



Why Is The Oxidation Number Of Oxygen In Hydrogen Peroxide 1 Quora
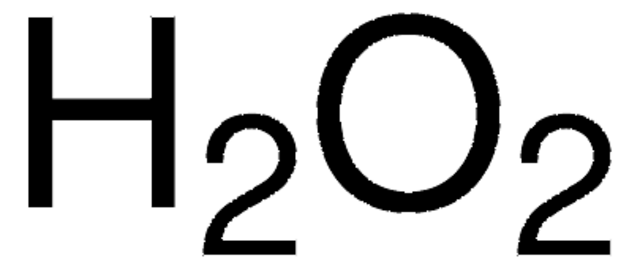


Hydrogen Peroxide 30 W W H2o Contains Stabilizer 7722 84 1 Sigma Aldrich
· Hydrogen peroxide, H 2 O 2, is a simple peroxide compound Other inorganic peroxides (aside from hydrogen peroxide) are known These are classified as either ionic peroxides or covalent peroxides Ionic peroxides contain alkali · Decide the proper grade you want to reach after mixing Keep in mind 3% Hydrogen Peroxide is the kind used to treat wounds and sanitize 1 It tends to need preservatives 6% Hydrogen Peroxide is typically used for hair coloring and sanitizing 1 30% ReAgent Hydrogen Peroxide is used in medical research while the 3032% Electronic Grade Hydrogen Peroxide isFormula Weight 0 100 (61) 101 0 (52) 1 300 (25) 301 400 (15) 401 500 (11) 501 600 (5) Structure Search AntiBone Sialoprotein II Antibody Intracellular Hydrogen Peroxide Assay 1 Product Result Match Criteria Product Name, Property, Description



Hydrogen Peroxide Lewis Dot Novocom Top


Hydrogen Peroxide How To Make Hydrogen Peroxide Uses Properties By Chemistry Page Medium
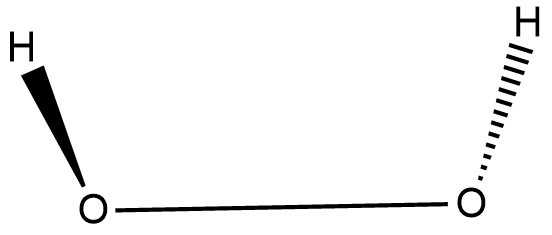
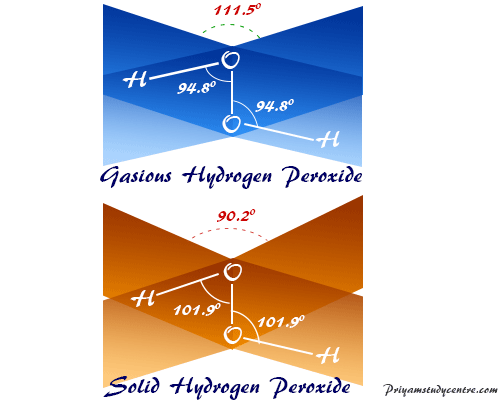
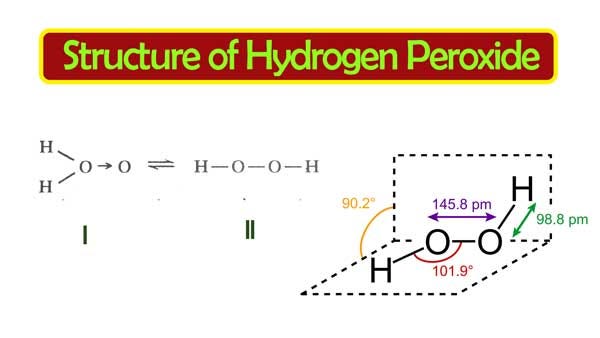
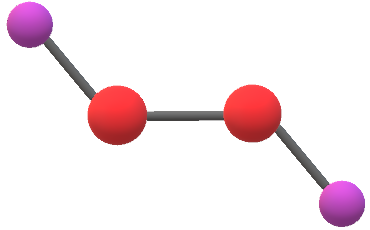
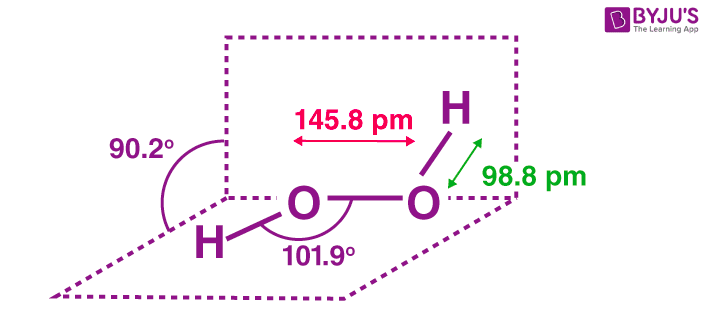
H 2 O 2 has a nonpolar structure The molecular dimensions in the gas phase and solid phase differ as shown in figure 45 Structurally, H 2 O 2 is represented by the dihydroxyl formula in which the two OHgroups do not lie in the same plane One way of explaining the shape of hydrogen peroxide is that the hydrogen atoms would lie on the pages of a partly opened book, and the3 Water — Hydrogen peroxide is a naturally occurring substance Surface water concentrations of hydrogen peroxide have been found to vary between mg/L, increasing both with exposure to sunlight and the presence of dissolved organic matter (IARC 1985) 4 Biota — Hydrogen peroxide is a naturally occurring substance Endogenous hydrogenStructural Formula H 2 O 2 hydrogen peroxide



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Royalty Free Cliparts Vectors And Stock Illustration Image
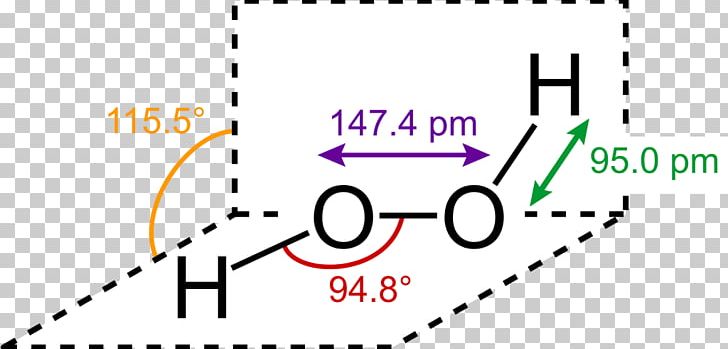


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png Clipart Angle Brand Chemical Bond Chemical Compound Chemical
Hydrogen peroxide forms a hydrate with water as H 2 O 2H 2 0 Why boiling point of hydrogen peroxide is higher than water?The chemical formula of hydrogen peroxide is H 2 O 2 which looks similar to water Its properties however, are completely different Hydrogen peroxide is commonly used as an antiseptic and to lighten or bleach hair Hydrogen peroxide is commonly sold · Hydrogen Peroxide consists of 2 hydrogens and 2 oxygen atoms arranged in an open book like structure with bent OHO bonds The electronegativity of oxygen is around 344 and that of hydrogen is 22 The difference between the electronegativity of O and H atoms causes the OH bond to be polar


Hydrogen Peroxide Formula Production Facts Uses Priyamstudycentre


Reaction Of The Breakdown Of Hydrogen Peroxide And Its Features Mel Chemistry



Hydrogen Peroxide Formula H2o2 Over 100 Million Chemical Compounds Mol Instincts



Hydrogen Peroxide Structure H2o2 Over 100 Million Chemical Compounds Mol Instincts



Why Hydrogen Peroxide Is A Polar Molecule Yvbi2pxx Chemistry Topperlearning Com
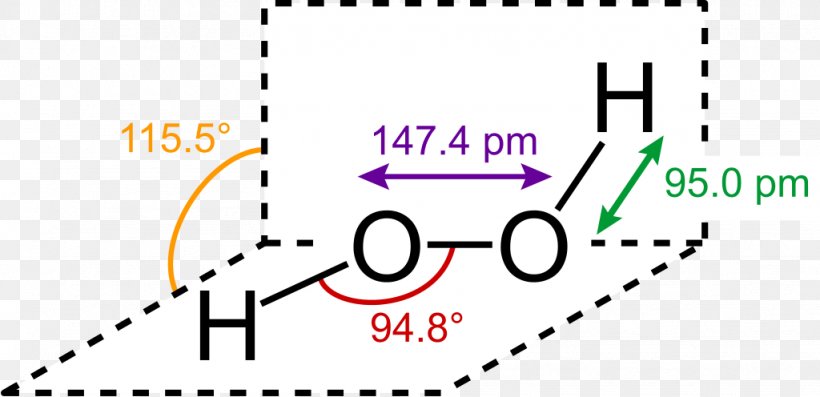


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png 1024x496px Lewis Structure Area Brand Chemical Bond Chemical


Hydrogen Peroxide Chemistry Class 11 Hydrogen



Hydrogen Peroxide Formula H2o2 Latest Price Manufacturers Suppliers


What Elements Are Present In Hydrogen Peroxide Quora



An Introduction To Reactive Oxygen Species Measurement Of Ros In Cells April 7 21


The Msds Hyperglossary Peroxide


Vector Ballandstick Model Of Chemical Substance Icon Of Hydrogen Peroxide Molecule H2o2 Consisting Of Hydrogen And Oxygen Structural Formula Suitable For Education Isolated On A White Background Stock Illustration Download Image



Hydrogen Peroxide H2o2 Stock Illustration Illustration Of Background


Illustrated Glossary Of Organic Chemistry Peroxide



Figure 1 From Aquaporin Facilitated Transmembrane Diffusion Of Hydrogen Peroxide Semantic Scholar


H2o2 Hydrogen Peroxide Molecule Royalty Free Vector Image


Nebraska Redox Biology Center Educational Portal


Hydrogen Peroxide H2o2 Chemspider



24 Molecular Formula Of Hydrogen Peroxide Stock Photos Pictures Royalty Free Images



Hydrogen Peroxide Water H4o3 Pubchem


Hydrogen Peroxide How To Make Hydrogen Peroxide Uses Properties By Chemistry Page Medium



Hydrogen Per Oxide Formula H2o2 Tpl Hydrogen Peroxide ह यड र जन प र क स इड In Delhi Chemical Suppliers Id


Carbamide Peroxide Ch6n2o3 Pubchem
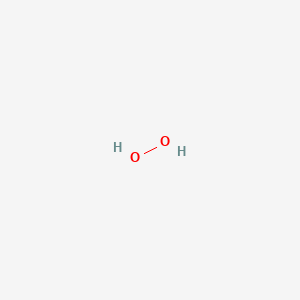


Hydrogen Peroxide H2o2 Pubchem
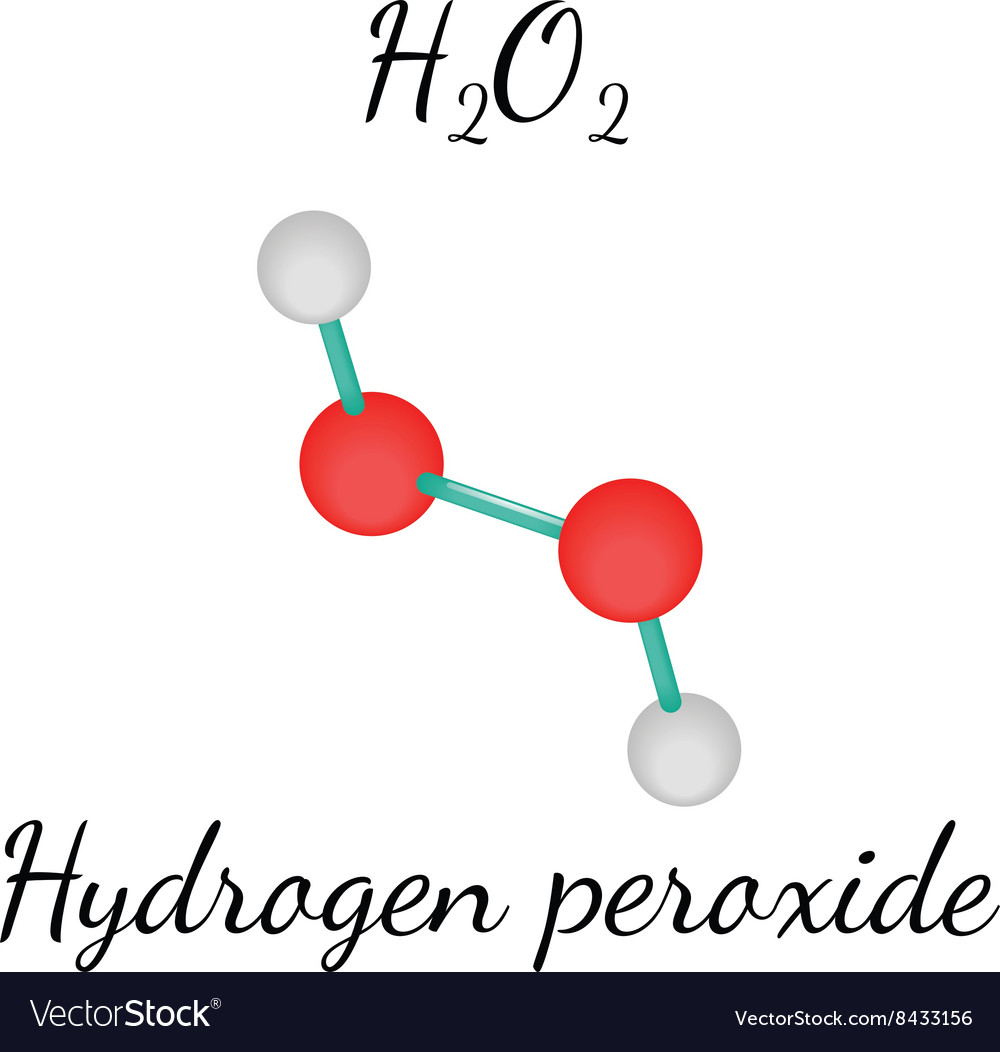


H2o2 Hydrogen Peroxide Molecule Royalty Free Vector Image
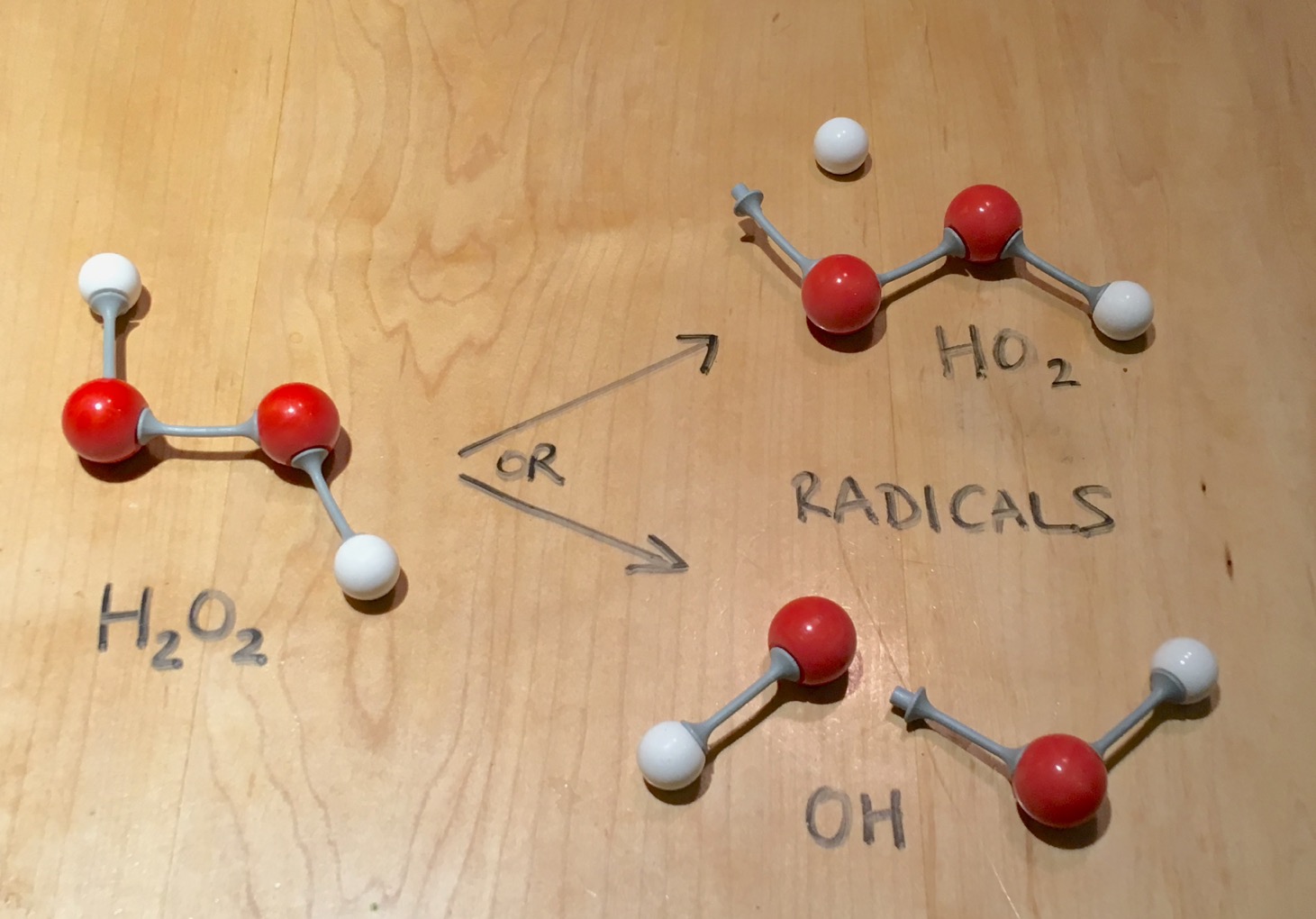


Hydrogen Peroxide Chemistry Ingridscience Ca
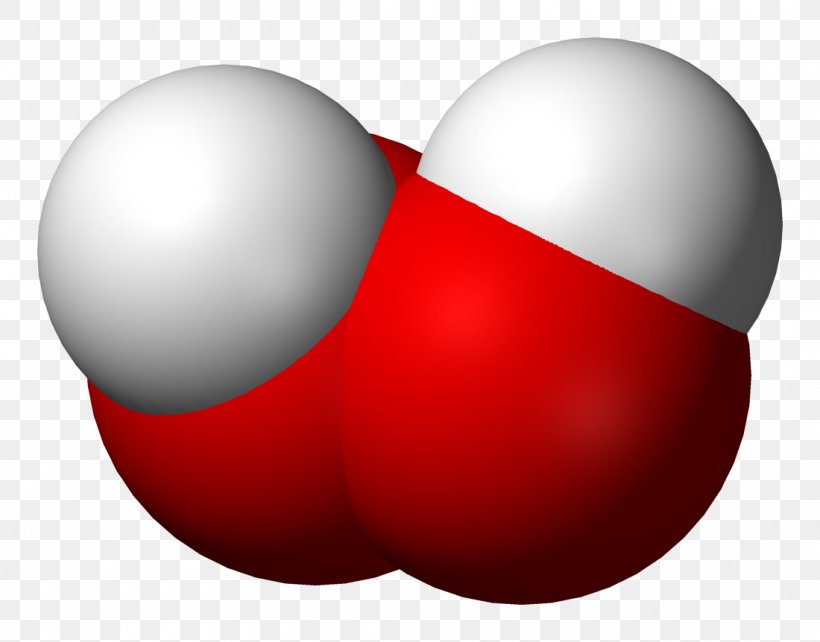


Hydrogen Peroxide Molecule Chemical Compound Oxygen Png 1100x862px Hydrogen Peroxide Chemical Compound Chemical Formula Chemistry Composto


Hydrogen Peroxide 7722 84 1



Hydrogen Peroxide H2o2 Molecular Structure Isolated On White Stock Illustration Illustration Of Hair Atom


Benzoyl Peroxide Benzoyl Group Chemical Formula Hydrogen Peroxide Png Clipart Acne Adapalenebenzoyl Peroxide Angle Area Benzoyl
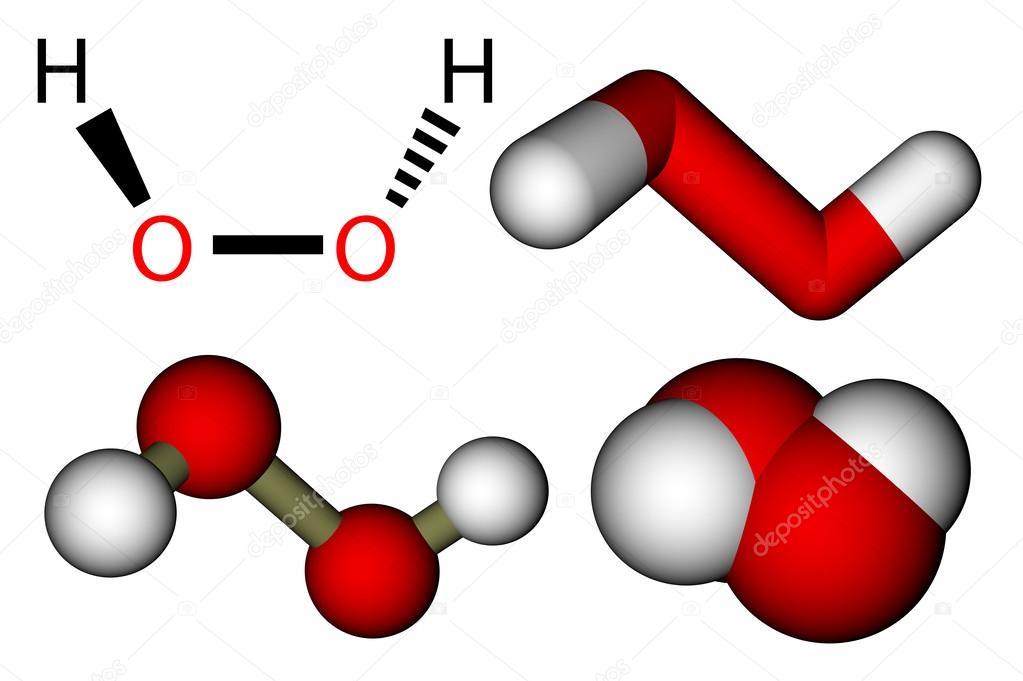


Hydrogen Peroxide H2o2 Structural Formula And 3d Molecular Mod Stock Photo Image By C Leonid Andronov



Hydrogen Peroxide Another Deadly Alternative The Chronicle Flask



Humidity In H2o2 Rotronic Usa
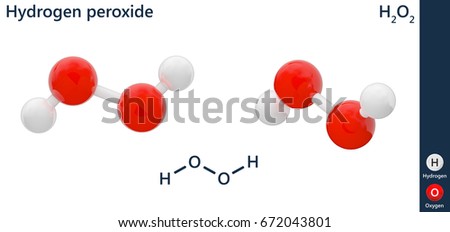


Hydrogen Peroxide Molecule Health
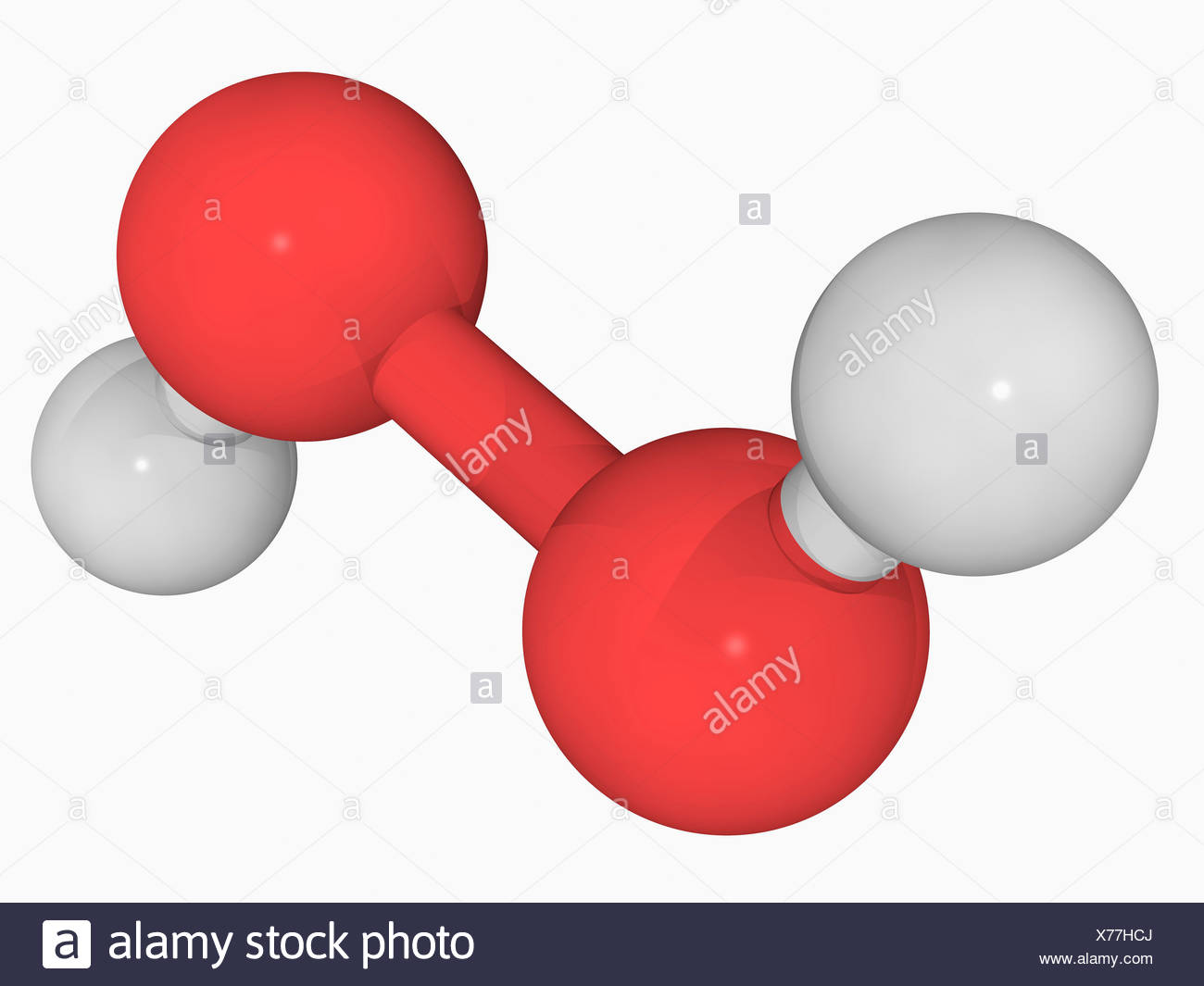


Hydrogen Peroxide High Resolution Stock Photography And Images Alamy



Intro To H2o2 Hydrogen Peroxide And You Bodyquirks



Hydrogen Peroxide Molecule Structure Stock Vector Royalty Free
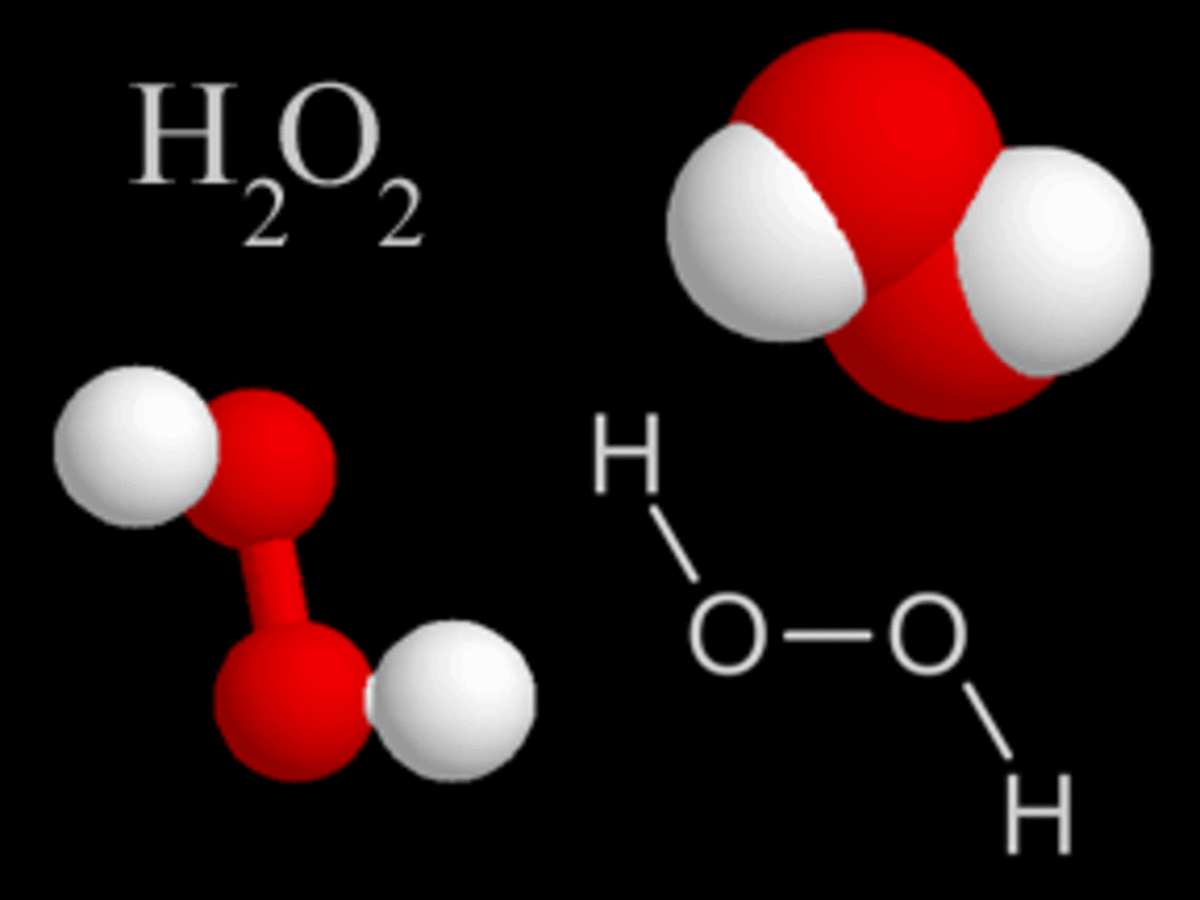


Hydrogen Peroxide Molecule Somor



Hydrogen Peroxide Molecule Ball And Stick Model Stock Footage Video 100 Royalty Free Shutterstock



Hydrogen Peroxide Wikipedia



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube
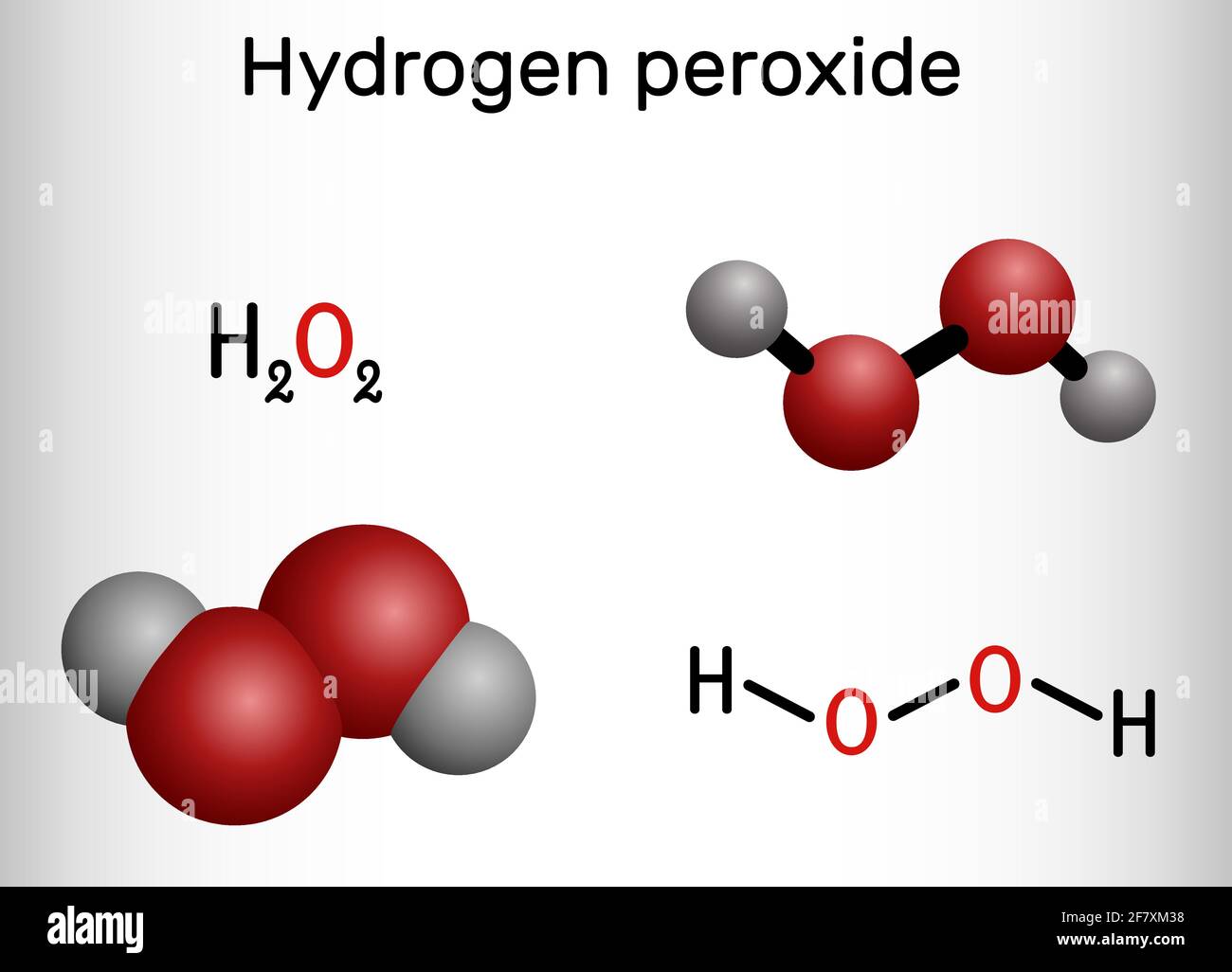


Hydrogen Peroxide High Resolution Stock Photography And Images Alamy
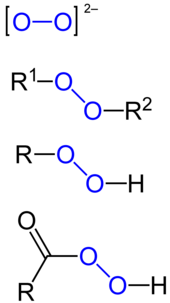


Peroxide Wikipedia



Learn Properties And Structure Of Hydrogen Peroxide In 3 Minutes


Hydrogen Peroxide Molecule Reactive Oxygen Species Ros Used As Bleaching Agent Disinfectant Chemical Reagent Etc Skeletal Stock Vector Illustration Of Molecular Reagent


Hydrogen Peroxide Molecule Structure Stock Vector Royalty Free



Hydrogen Peroxide Health Safety Tips Msdsonline



How To Write The Formula For Hydrogen Peroxide Youtube



Draw The Lewis Structure For Hydrogen Pero Clutch Prep


Hydrogen Peroxide Structure Uses And Properties Of Hydrogen Peroxide



Lewis Structure Hydrogen Peroxide Molecule Structural Formula Laughing Gas Structure Angle Text Png Pngegg



Chemical Makeup Of Hydrogen Peroxide Saubhaya Makeup


What Is The Empirical Formula Of H2o2 Socratic


H2o2 Lewis Structure Hydrogen Peroxide Molecular Geometry Polarity



Pdf Empirical Formula Of Hydrogen Peroxide



Hydrogen Peroxide



Molecular Facts And Structures Chemical Structure Molecular Lettering
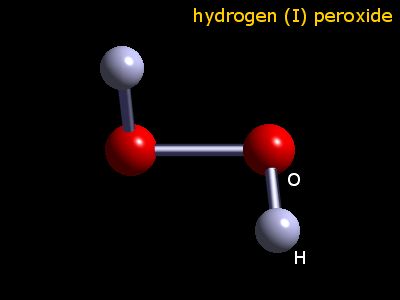


Webelements Periodic Table Hydrogen Hydrogen Peroxide


Liquid Explosives Review



Vsepr H2o2 Lewis Structure Novocom Top
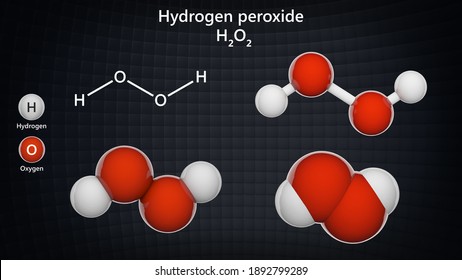


Hydrogen Peroxide Molecule High Res Stock Images Shutterstock


Peroxide Wikipedia
/hydrogen-peroxide-molecule-147216853-5c719552cff47e0001b1e31a.jpg)


10 Dangerous Chemicals You Should Avoid



Hydrogen Peroxide Chemical Equation Health


Hydrogen Peroxide Formula



15 Hydrogen Peroxide Structural Formula Stock Photos Pictures Royalty Free Images
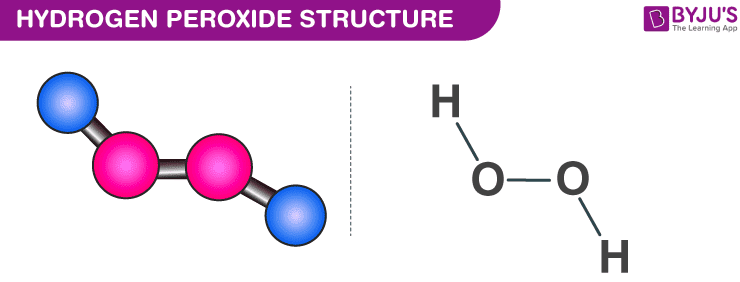


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses
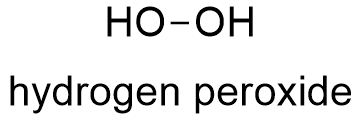


Hydrogen Peroxide Formula


Hydrogen Peroxide Molecule Of The Month September 06 Html Version
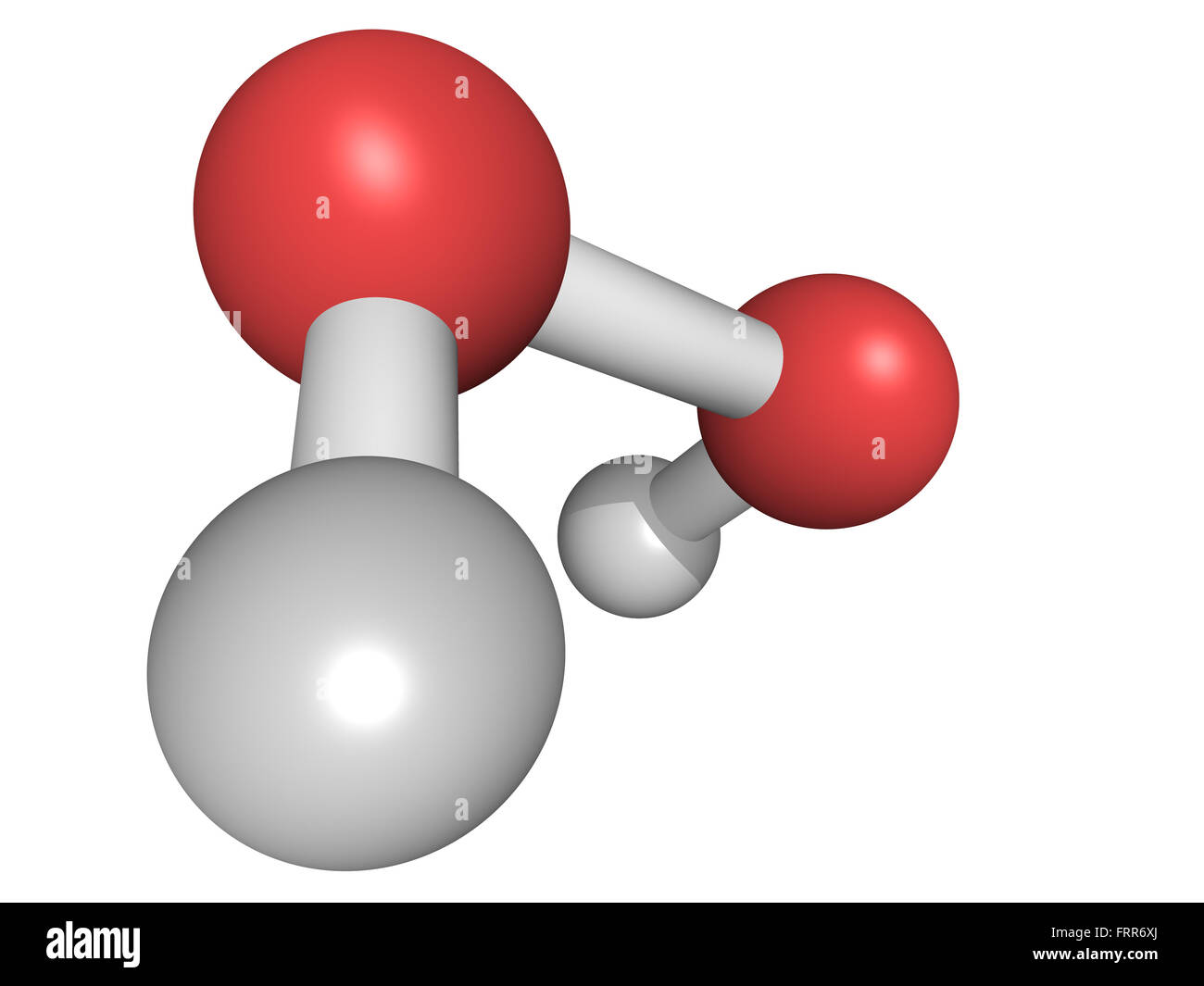


Hydrogen Peroxide High Resolution Stock Photography And Images Alamy
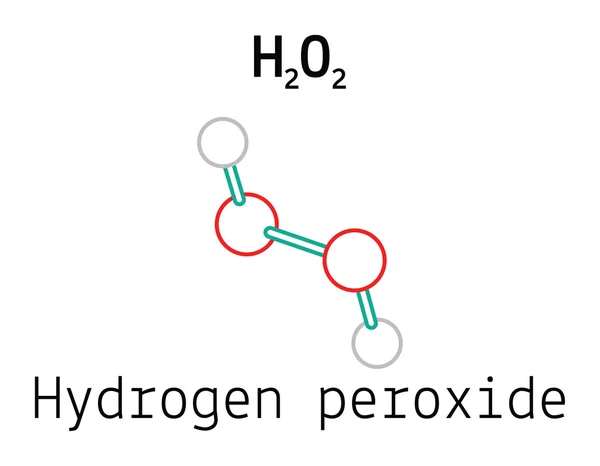


92 Hydrogen Peroxide Vectors Royalty Free Vector Hydrogen Peroxide Images Depositphotos


16 5b Hydrogen Peroxide H 2o 2 Chemistry Libretexts
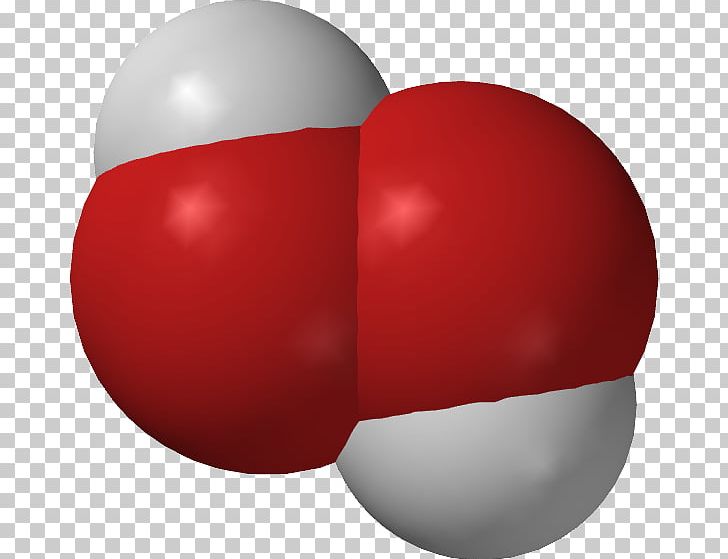


Hydrogen Peroxide Space Filling Model Molecular Model Molecule Png Clipart Barium Peroxide Bohr Model Chemical Compound



Urea Hydrogen Peroxide 97 124 43 6 Sigma Aldrich


Is Benzoyl Peroxide The Same As Hydrogen Peroxide Quora



Lewis Structure Of Hydrogen Peroxide H2o2 Novocom Top


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses



Hydrogen Peroxide Uses And Effects I Curious Chloride Scans Product S Ingredients
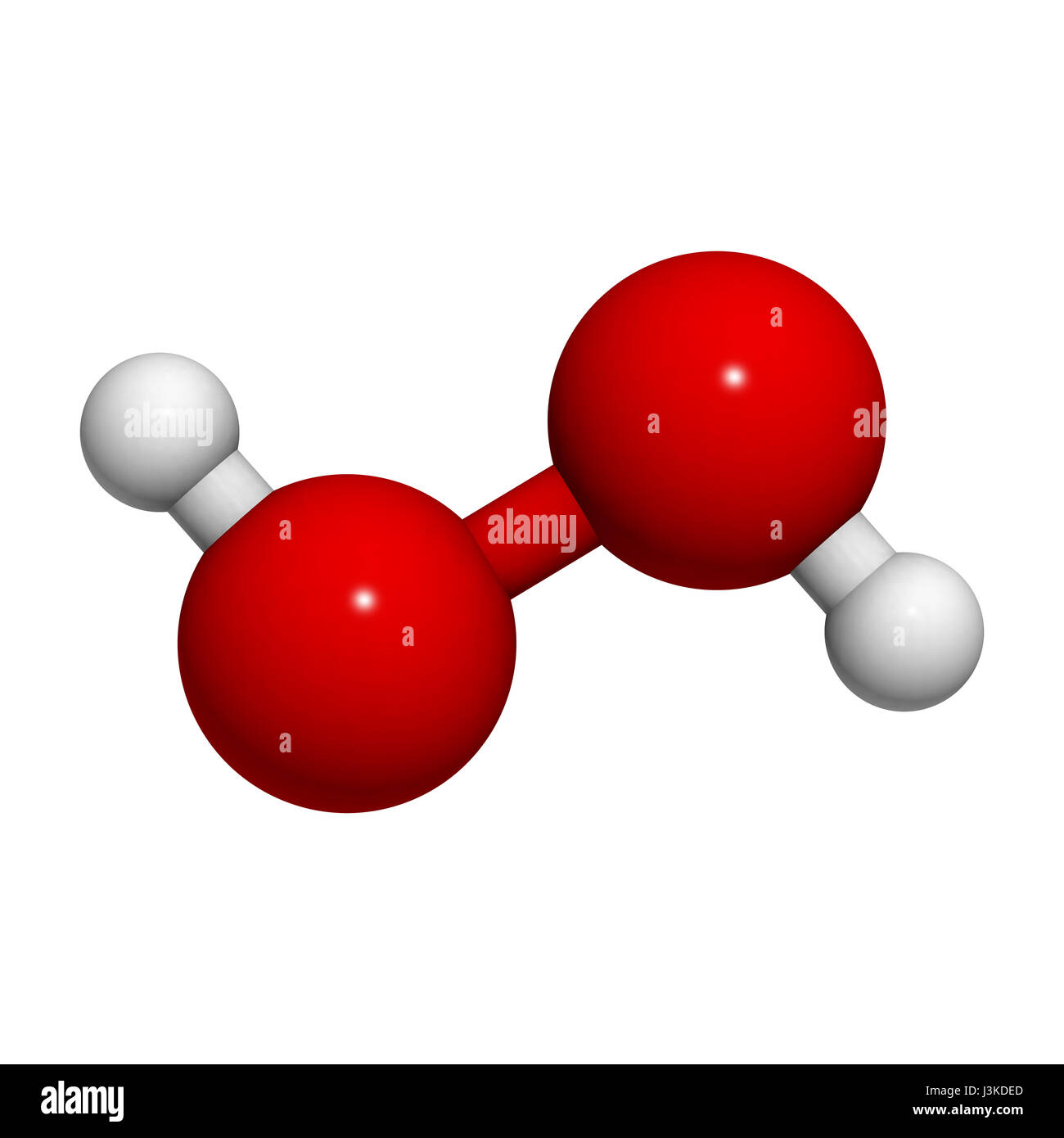


Hydrogen Peroxide High Resolution Stock Photography And Images Alamy



Hydrogen Peroxide Simple English Wikipedia The Free Encyclopedia



Sulfur Dioxide Lewis Structure Molecule Molecular Geometry Resonance Silicon Dioxide Structure Angle Text Chemistry Png Pngwing



H2o2 Search Larastock Stock Photos Royalty Free Images Vectors



0 件のコメント:
コメントを投稿